Subscribe to the Interacoustics Academy newsletter for updates and priority access to online events
Training in VNG
-
Saccadometry in Clinical Practice
-
BSA Guidance for Eye Movement Recordings (2015)
-
BSA Guidance for Positioning Tests (2016)
-
Eye Movements with Progressive Supranuclear Palsy (PSP)
-
Cutoff for Normal vs Abnormal OCR Response
-
Significance of Upbeat Nystagmus in Smokers
-
How Large are Significant Square Wave Jerks?
-
Does Repositioning of Otoconia Affect Utricular Function?
-
How to Differentiate Between Spinocerebellar Ataxia Subtypes Using VNG
-
When is Torsional Nystagmus Significant?
-
Skew Deviation in HINTS Protocol: Next Step?
-
Course: Advances in Videonystagmography (VNG)
-
Course: Advances in BPPV
-
Course: Balance Testing for Beginners
-
Course: Balance Testing for Intermediates
-
What is the Optokinetic Nystagmus Test?
-
What is the Saccade Test?
-
BPPV: Case Studies
-
Benign Paroxysmal Positional Vertigo (BPPV): An Introduction
-
Otolith Testing: Subjective Visual Vertical
-
Otolith Testing: Ocular Counter Roll Test
-
Using the TRV Chair for BPPV Treatment
-
Physiology of the Otolith Organs
-
Diagnosing Central Vestibular Conditions with VNG
-
3D Eye Movement Recordings for BPPV Testing
-
Otolith Testing in VNG
-
The Importance of Vertical and Torsional Eye Movements in the Diagnosis of Vestibular and Neurological Conditions
-
VNG: Gaze Testing Without Fixation
-
VNG: Gaze Testing With Fixation
-
Oculomotor Testing: Theoretical Introduction
-
How to Diagnose Lateral Canal BPPV
-
Course: Saccadometry – a new tool for assessing central vestibular disorders
-
Course: Diagnosing Peripheral Vestibular Disorders
-
Course: The role of repositioning chairs in the diagnosis and treatment of BPPV
-
Course: Which test, when: Exploring optimal vestibular assessment protocol through illustrative case studies
-
Performing calibration for VNG testing
-
Performing spontaneous nystagmus testing (VNG)
-
Performing the gaze test (VNG)
-
Performing smooth pursuit testing (VNG)
-
Performing saccade testing (VNG)
-
Performing optokinetic testing (VNG)
-
Performing Dix Hallpike testing (VNG)
-
Performing positional testing (VNG)
-
Getting started: VNG
-
Understanding torsional eye movements
-
Interpreting the components of protocols that use torsional analysis in VisualEyes™
-
Interpreting the Dizziness Handicap Inventory (DHI) and VRBQ
-
Understanding Jerk Nystagmus
-
The role of eye movements in measuring brain function
-
BPPV: the importance of torsion and performing the manoeuvres accurately
-
Diagnostic driven rehabilitation strategies for concussion and other disorders
-
Physiology of torsional eye movements
-
Measuring torsional eye movements
-
The importance of measuring torsion
-
The role of audiology in concussion diagnostics
-
Eye movements in neuroperformance: A brain focused assessment of eye movements
-
Mise en route: VNG
-
Beyond the basics: Identifying and minimizing artifacts in VNG
-
VPPB: Una Introducción
-
Vidéonystagmographie (VNG) : une introduction
-
Effectuer un test de saccade (VNG)
-
Effectuer le test optocinétique (VNG)
-
Effectuer un test Dix Hallpike (VNG)
-
Effectuer un étalonnage pour les tests VNG
-
Effectuer des tests de position (VNG)
-
Effectuer un test de nystagmus spontané (VNG)
-
Effectuer un test du regard (VNG)
-
Effectuer des tests de poursuite lente (VNG)
-
VNG pruebas oculomotoras
-
Erste Schritte: Video-Nystagmographie (VNG)
-
Kalibration der Augenpositionen
-
Einführung VNG
-
Blickrichtung / Blickstabilisierung Test
-
Spontan Nystagmus
-
Saccaden Test
-
Glatte Blickfolge Test
-
Optokinetik Test
-
Lage-/Lagerungstest
-
Dix-Hallpike Test
-
Kalorik-Test
Saccadometry: An introduction
Description
Table of contents
- What is Saccadometry?
- Integrating Saccadometry into clinical practice
- Performing Saccadometry
- Reviewing the results
- Clinical applications of Saccadometry
What is Saccadometry?
Saccadometry is an advanced and non-invasive oculomotor test that provides functional insights into the integrity of the brain regions and neural circuits involved in generating saccadic eye movements.
Unlike traditional saccade testing, which has traditionally been limited to numerical measurements of latency, amplitude, and velocity, Saccadometry delivers a more comprehensive neurological evaluation by incorporating latency distribution, phase metrics, position distribution, and error detection. This is also enhanced further by the addition of a cognitive reflex inhibition task when performing the antisaccade assessment (Munoz & Everling, 2003).
Saccadometry is proving increasingly useful in the diagnosis and management of a wide range of conditions, including:
- Movement disorders
- Mood disorders including depression
- Concussion and traumatic brain injury (TBI)
- Attention-deficit/hyperactivity disorder (ADHD)
- Neurodegenerative diseases such as Parkinson’s and Alzheimer’s
The video below summarizes what Saccadometry is and what it brings to the clinic.
Integrating Saccadometry into clinical practice
Clinics can seamlessly integrate Saccadometry into their diagnostic and assessment protocols to enhance the evaluation of neurological and cognitive function. With Saccadometry being incorporated into VisualEyes™ VNG, you can perform Saccadometry in the clinic as part of routine neurological exams or specialized assessments.
By adding Saccadometry to existing workflows, clinicians gain access to objective, quantitative data on the functional status of key brain regions. This allows for more accurate diagnosis, earlier detection of subtle dysfunctions, and better tracking of treatment outcomes.
In the video below, Dr. Michelle Petrak, Ph.D., will provide her insights into how and why she has incorporated Saccadometry into her clinic workflow.
Performing Saccadometry
The protocols are performed using the VisualEyes VNG system. The oculomotor stimuli are projected onto a large TV screen. The patient is seated in front of the screen. The background of the screen is kept black, and this is because high contrast screens are difficult for these patients to tolerate for the extended duration of the Saccadometry test.
The default protocol settings are listed in Table 1.
| Parameter | Setting |
| Duration | 151 seconds |
| Number of jumps | 60 |
| Jump size | 10 degrees |
| Mean time interval | 1.5 seconds |
| Number of dots | 2 |
| Artifact rejection | On |
| Jump pattern | Randomized |
| Compensate for drift | On |
| Target colors | Red, Red, Red |
| Background color | Black |
| Enable practice mode | Off |
Table 1: Default Saccadometry protocol settings in VisualEyes™.
Prosaccades
As with traditional oculomotor testing, instruct the patient to keep their head still and follow the target movement with their eyes only.
For prosaccades, instruct the patient to:
- Look at the center dot
- For each saccade jump, look at the new target
- And then return to the center dot and wait for the next jump
We can look at this assessment in action in the following video. Darren Whelan will explain the process from calibration to patient instructions, to performing the assessment, and finally reviewing and validating the data recorded.
Antisaccades
For the antisaccades, instruct the patient to look in the opposite direction of the saccade jump and then return to the center and wait for the next jump.
We can look at this assessment in action in the following video. Darren Whelan will explain the process from calibration to patient instructions, to performing the assessment, and finally reviewing and validating the data recorded.
Reviewing the results
Now that we have seen both the prosaccade and antisaccade assessments, we can look at the data in more detail. The graphs below illustrate the patient’s eye movements relative to the target movements.
The target movement is indicated by the yellow trace and the patient’s eye movements are indicated as a red trace for the right eye and blue trace for the left eye.
In the prosaccades, you can see the subject looks toward the target jumps. In the antisaccades, they are looking away from the target jumps.
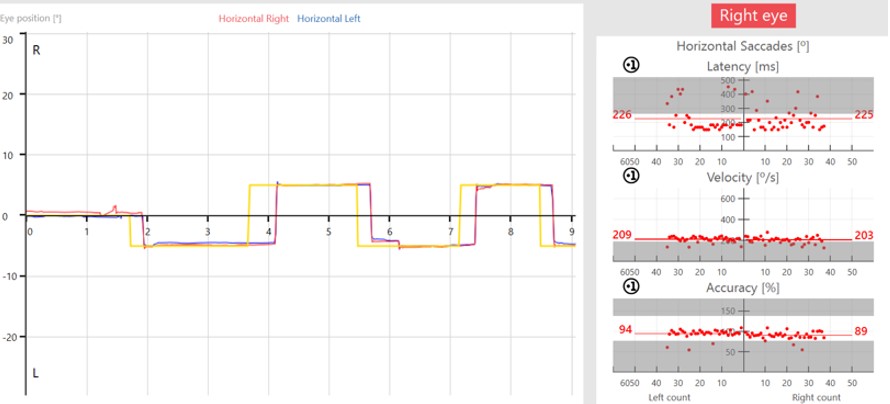
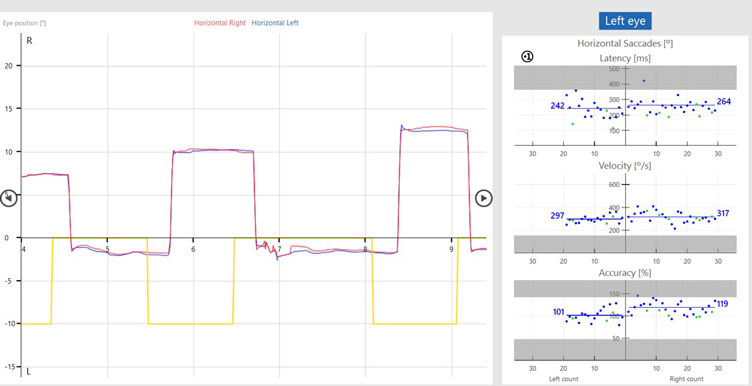
Saccade parameters
As discussed earlier, we are assessing the saccade parameters of latency, velocity, and accuracy, which are the same as what we examine in the standard random saccade test. What is different is once the test is complete, we can see summary graphs for:
- Position
- Velocity
- Latency
- Phase
- Error rate
If we look at error rate as an example, there are two measurements under error rate:
- Directional error rate
- Overall error rate
Directional error rate is the percentage of times the patient moves their eyes in the incorrect direction. The overall error rate is an indication of overall noise and artifact present in the recording, so we can think of this as a quality check.
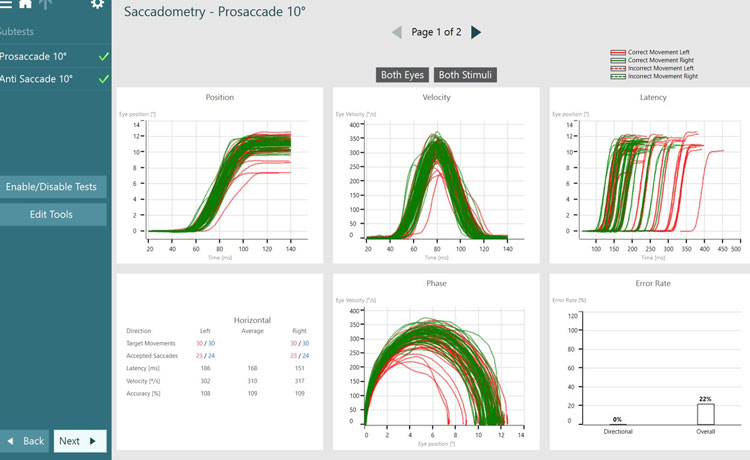
The green lines represent targets moving to the right, with eye movements associated with that target position being controlled by the left side of the brain. The red lines represent targets moving to the left, with eye movements associated with that target position being controlled by the right side of the brain.
Dotted lines represent eye jumps where the patient looked in the incorrect direction, hence causing an error to be recorded (see Figures 4 and 5). After a completed test, you can also visualize the data by stimulus direction. This can aid in a more detailed diagnosis and help to distinguish the side of the lesion.
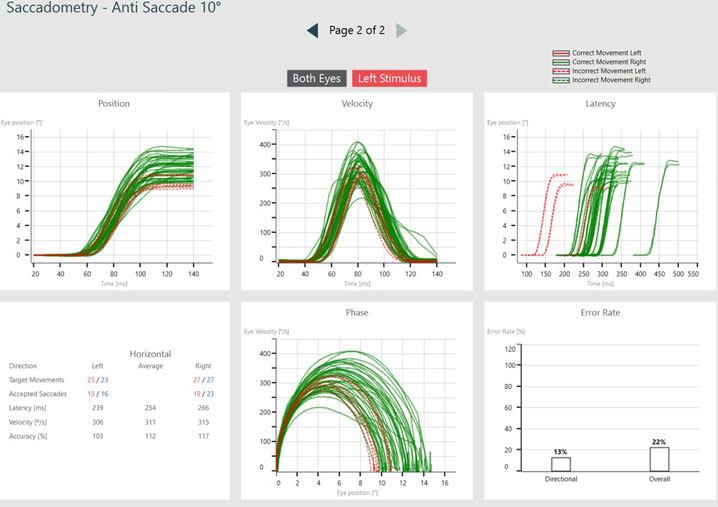
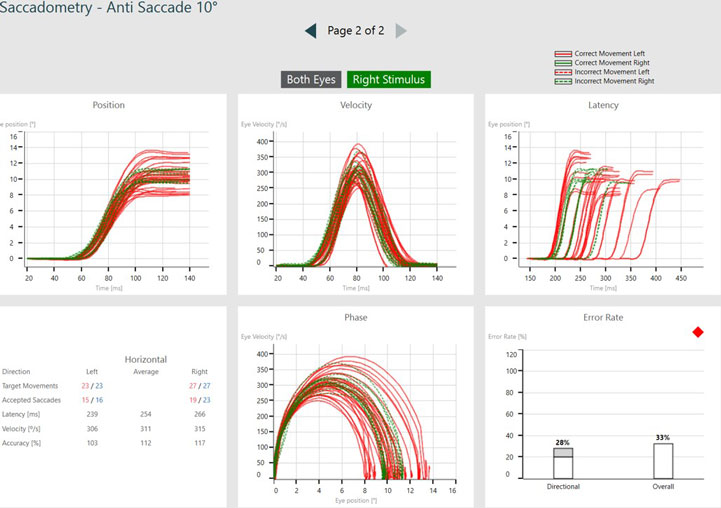
Saccadometry provides a lot more detailed information, and it can take a moment to navigate the graphs and data representations. We can take a guided tour through the summary of Saccadometry data with Dr. Elizabeth Fuemmeler, Au.D.
Clinical applications of Saccadometry
Below, we will cover the many exciting clinical applications of Saccadometry, as shown in the published research.
1. Inhibitory control and voluntary motor execution
The ability to suppress automatic, reflexive responses in favor of voluntary actions is essential for functioning in daily life. The antisaccade test is a widely used tool to evaluate both inhibitory control and voluntary motor execution. During this task, patients must suppress the instinctive urge (reflex) to look toward a sudden visual stimulus (prosaccade) and instead direct their gaze in the opposite direction.
Research has shown that individuals with various brain lesions, neurological disorders, and psychiatric conditions often struggle with this task, frequently making directional errors by reflexively looking toward the stimulus. These prosaccade errors reflect deficits in response inhibition and cognitive control.
Neuroimaging studies have identified a broad network of cortical and subcortical brain regions involved in antisaccade generation, including the frontal eye fields, prefrontal cortex, supplementary eye fields, and basal ganglia.
Dysfunction in any part of this network can contribute to impaired antisaccade performance, making this test a valuable tool in the assessment of brain health (Ting et al., 2016; Muñoz et al., 2004; Termsarasab et al., 2015; Rodrigue et al., 2016; Heuer et al., 2013; Yang et al., 2013; Hoffmann et al., 2019; Jensen et al., 2019; Beck et al., 2018; Shaikh et al., 2019).
2. Sensory integration and spatial localization
A core function of the human brain is to continuously determine the body’s position within the environment, which is a prerequisite for executing appropriate motor and behavioral responses. This process relies on the integration of three primary sensory systems (Table 2).
| Sensory system | Purpose |
| Proprioceptive system | Provides real-time feedback on the mechanical status of muscles, tendons, and joints. |
| Vestibular system | Informs the brain about head position relative to gravity, as well as the direction and velocity of head movements. |
| Visual system | Offers spatial information regarding body orientation and movement in relation to the external visual environment. |
Table 2: Human sensory systems.
Together, these systems contribute to postural control, balance, and navigation. While numerous visual reflexes support spatial orientation, accurate localization is critically dependent on the ability to perform rapid and precise saccadic eye movements. Saccades allow the brain to efficiently scan and map the visual field, enabling timely sensory-motor coordination and situational awareness.
Neuroimaging and clinical studies have shown that saccadic control involves widespread activation across cortical and subcortical networks, including the superior colliculus, frontal eye fields, parietal cortex, and cerebellum (McDowell et al., 2008; Leigh & Zee, 2015). These systems interact closely with vestibular and proprioceptive inputs to maintain orientation and gaze stability during movement (Angelaki & Cullen, 2008).
3. Localizing neurological lesions
Saccadic eye movements rely on the occipital lobe for visual processing, the parietal lobe for spatial localization, and the frontal lobe for initiating voluntary saccades (Pierrot-Deseilligny et al., 2004).
Once a saccade is initiated, neural signals descend from the frontal cortex through the basal ganglia, which modulate motor control and movement selection. These pathways project to the brainstem, where the superior colliculus and associated saccadic pulse generators coordinate commands to the oculomotor nuclei to move the eyes toward the target (Munoz & Schall, 2004; McDowell et al., 2008).
The cerebellum plays a vital role in calibrating saccades by adjusting amplitude and timing to ensure accurate targeting. This cerebellar feedback is essential for adaptive control and correction of eye movements (Robinson & Fuchs, 2001; Takagi et al., 1998).
Critically, different patterns of saccadic dysfunction can help clinicians localize neurological lesions (Table 3).
| Saccadic dysfunction | Indication |
| Delayed or absent saccades | Frontal lobe or basal ganglia involvement |
| Hypometric or hypermetric saccades | Cerebellar dysfunction |
| Directional errors (especially in antisaccade tasks) | Prefrontal or parietal deficits |
Table 3: Using patterns of saccadic dysfunction to localize neurological lesions (Leigh & Zee, 2015; Gooding & Basso, 2008).
4. Post-concussion
The distributed nature of pathways and structures involved in prosaccade and antisaccade generation makes them very sensitive to damage in concussion and traumatic brain injury (Ting et al., 2016). Increased latencies and expanded latency distributions have been shown to be sensitive signs of unresolved mTBI.
5. Movement disorders
Aberrant patterns of saccade generation are well-represented in movement disorder literature. Changes in saccade phase can help localize lesions within the frontal-basal ganglia network. Changes in accuracy can reveal aberrancies in the superior collicular saccade maps that are crucial to appropriate motor output in movement disorders such as Dystonia (Beck et al., 2018) and Parkinson’s disease (Shaikh et al., 2019).
These parameters, when combined with directional changes in saccade velocity, can aid in appropriate diagnosis of Parkinsonian syndromes such as Progressive Supranuclear Palsy and Multiple System Atrophy (Termsarasab et al., 2015).
6. Executive function disorders
Relationships between prosaccades and antisaccades can also provide insight into executive function disorders ranging from Mild Cognitive Impairment to Alzheimer’s disease and Frontotemporal Dementia (Heuer et al., 2013).
Summary
By integrating Saccadometry, clinicians can deliver high-precision diagnostics and advanced functional monitoring, positioning assessment and clinical practice at the forefront of evidence-based care.
Related courses
References and further reading
Angelaki, D. E., & Cullen, K. E. (2008). Vestibular system: the many facets of a multimodal sense. Annual review of neuroscience, 31, 125–150.
Beck, R. B., Kneafsey, S. L., Narasimham, S., O'Riordan, S., Isa, T., Hutchinson, M., & Reilly, R. B. (2018). Reduced Frequency of Ipsilateral Express Saccades in Cervical Dystonia: Probing the Nigro-Tectal Pathway. Tremor and other hyperkinetic movements (New York, N.Y.), 8, 592.
Carrick, F. R., Hankir, A., Zaman, R., Antonucci, M. M., Pagnacco, G., Azzolino, S., & Oggero, E. (2019). Improvement of Saccadic Eye Movements after Head-Eye Vestibular Motion (HEVM) Therapy and Neuro-Psychiatric Considerations. Psychiatria Danubina, 31(Suppl 3), 318–323.
Gooding, D. C., & Basso, M. A. (2008). The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain and cognition, 68(3), 371–390.
Heuer, H. W., Mirsky, J. B., Kong, E. L., Dickerson, B. C., Miller, B. L., Kramer, J. H., & Boxer, A. L. (2013). Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology, 81(14), 1235–1243.
Hoffmann, A., Ettinger, U., Montoro, C., Reyes Del Paso, G. A., & Duschek, S. (2019). Cerebral blood flow responses during prosaccade and antisaccade preparation in major depression. European archives of psychiatry and clinical neuroscience, 269(7), 813–822.
Jensen, K., Beylergil, S. B., & Shaikh, A. G. (2019). Slow saccades in cerebellar disease. Cerebellum & Ataxias, 6(1), Article 1.
Ting, W. K., Schweizer, T. A., Topolovec-Vranic, J., & Cusimano, M. D. (2016). Antisaccadic Eye Movements Are Correlated with Corpus Callosum White Matter Mean Diffusivity, Stroop Performance, and Symptom Burden in Mild Traumatic Brain Injury and Concussion. Frontiers in neurology, 6, 271.
Leigh, R. J., & Zee, D. S. (2015). The neurology of eye movements (5th ed.). Oxford University Press.
McDowell, J. E., Dyckman, K. A., Austin, B. P., & Clementz, B. A. (2008). Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain and cognition, 68(3), 255–270.
Munoz, D. P., & Everling, S. (2004). Look away: the anti-saccade task and the voluntary control of eye movement. Nature reviews. Neuroscience, 5(3), 218–228.
Muñoz, D. P., & Schall, J. D. (2004). Concurrent, distributed control of saccade initiation in the frontal eye field and superior colliculus. In L. M. Chalupa & J. S. Werner (Eds.), The visual neurosciences (Vol. 2, pp. 1449–1462). MIT Press.
Pierrot-Deseilligny, C., Milea, D., & Müri, R. M. (2004). Eye movement control by the cerebral cortex. Current opinion in neurology, 17(1), 17–25.
Pinkhardt, E. H., & Kassubek, J. (2011). Ocular motor abnormalities in Parkinsonian syndromes. Parkinsonism & related disorders, 17(4), 223–230.
Robinson, F. R., & Fuchs, A. F. (2001). The role of the cerebellum in voluntary eye movements. Annual review of neuroscience, 24, 981–1004.
Rodrigue, A. L., Austin, B. P., Dyckman, K. A., & McDowell, J. E. (2016). Brain activation differences in schizophrenia during context-dependent processing of saccade tasks. Behavioral and brain functions : BBF, 12(1), 19.
Samadani, U., Ritlop, R., Reyes, M., Nehrbass, E., Li, M., Lamm, E., Schneider, J., Shimunov, D., Sava, M., Kolecki, R., Burris, P., Altomare, L., Mehmood, T., Smith, T., Huang, J. H., McStay, C., Todd, S. R., Qian, M., Kondziolka, D., Wall, S., … Huang, P. (2015). Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. Journal of neurotrauma, 32(8), 548–556.
Shaikh, A. G., & Ghasia, F. F. (2019). Saccades in Parkinson's disease: Hypometric, slow, and maladaptive. Progress in brain research, 249, 81–94.
Takagi, M., Zee, D. S., & Tamargo, R. J. (1998). Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. Journal of neurophysiology, 80(4), 1911–1931.
Termsarasab, P., Thammongkolchai, T., Rucker, J. C., & Frucht, S. J. (2015). The diagnostic value of saccades in movement disorder patients: a practical guide and review. Journal of clinical movement disorders, 2, 14.
Yang, Q., Wang, T., Su, N., Xiao, S., & Kapoula, Z. (2013). Specific saccade deficits in patients with Alzheimer's disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age (Dordrecht, Netherlands), 35(4), 1287–1298.
Presenter

Get priority access to training
Sign up to the Interacoustics Academy newsletter to be the first to hear about our latest updates and get priority access to our online events.
By signing up, I accept to receive newsletter e-mails from Interacoustics. I can withdraw my consent at any time by using the ‘unsubscribe’-function included in each e-mail.
Click here and read our privacy notice, if you want to know more about how we treat and protect your personal data.
